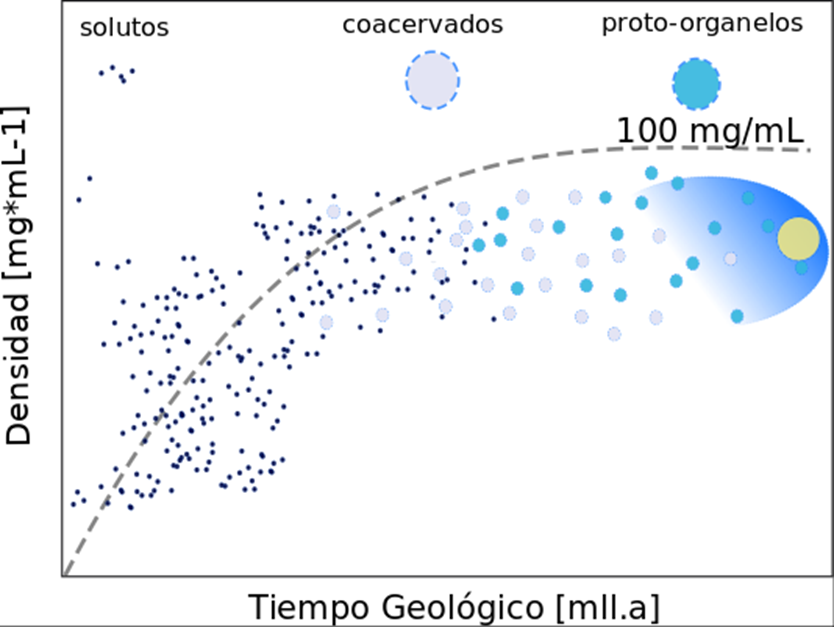
Orígenes físicos del citoplasma y la emergencia del código genético
Physical origins of the cytoplasm and the emergence of the genetic code
Mostrar biografía de los autores
El origen de la vida está ligado al origen del código genético, ningún organismo podría considerarse vivo si no posee la capacidad de transmitir y ejecutar instrucciones químicas replicables, mutables, variables y heredables a futuras generaciones. En esta revisión se aborda el problema de qué condiciones son necesarias para iniciar el origen de los bloques del código genético.
Además se discute el origen ancestral del citoplasma como una matriz bioquímica que soporta la replicación, síntesis y preservación del código genético. La discusión se centra sobre el origen de la vida con pruebas experimentales sobre la síntesis de ADN/ARN ex vivo, sino también con las descripciones fundamentales de los principios biológicos esenciales para el origen de los seres vivos.
Visitas del artículo 565 | Visitas PDF
Descargas
- Aran, M., Ferrer-Sueta, G., & Radi, R. (2011). ATP and Mg2+ promote the reversible oligomerization and aggregation of chloroplast 2-Cys periredoxin. The Journal of Biological Chemistry, 286(26), 23441-23451. doi: 10.1074/jbc.M111.224964
- Abbas, M., & Kakkar, V. (2021). Peptide-based coacervates as biomimetic protocells. Chemical Society Reviews, 50(7), 3690-3705. doi: 10.1039/d0cs00307g
- Arsene, S., Delcea, M., & Kelemen, L. (2018). Coupled catabolism and anabolism in autocatalytic RNA sets. Nucleic Acids Research, 46(18), 9384-9393. https://doi.org/10.1093/nar/gky699
- Arai, Y., Nomura, T., Sakuma, S., Nagai, T., & Urano, Y. (2018). RGB-color intensiometric indicators visualize spatiotemporal dynamics of ATP in single cells. Angewandte Chemie International Edition in English, 57(34), 10873-10878. https://doi.org/10.1002/anie.201804304
- Baev, A. Y., Cheryasov, G. V., & Skulachev, V. P. (2020). Inorganic polyphosphate is produced and hydrolyzed in F0F1-ATP synthase of mammalian mitochondria. Biochemical Journal, 477(8), 1515-1524. doi: 10.1042/BCJ20200141
- Brangwynne, C. P., Eckmann, C. R., Courson, D. S., Rybarska, A., Hoege, C., Gharakhani, J., … Hyman, A. A. (2011). Active liquid-like behavior of nucleoli determines their size and shape in Xenopus laevis oocytes. Proceedings of the National Academy of Sciences of the United States of America, 108(11), 4334-4339. https://doi.org/10.1073/pnas.1017150108
- Bose, T., Lee, K. H., Lu, S., Xu, J., & Zhang, Y. (2022). Liquid-to-solid phase transition of oskar ribonucleoprotein granules is essential for their function in Drosophila embryonic development. Cell, 185(8), 1308-1324. https://doi.org/10.1016/j.cell.2022.02.022
- Chandrasekhar, R., Melin, J., & Lindgren, A. (2019). A molecular sensor reveals differences in macromolecular crowding between the cytoplasms and nucleoplasm. ACS Sensors, 4(8), 1835-1843. https://doi.org/10.1021/acssensors.9b00569
- Chen, H., Zheng, X., Zheng, Y., & Anderson, D. H. (2019). Nucleoplasmin is a Limiting Component in the Scaling of Nuclear Size with Cytoplasmic Volume. Journal of Cell Biology, 218(12), 4063-4078. https://doi.org/10.1083/jcb.201908059
- Diaz-Delgadillo, A. F. (2016). Temperature drives P granule formation in C. elegans. Editorial: Sächsiches Library TUDresden.
- Diaz-Delgadillo, A. F. (2021). Local thermodynamics govern formation and dissolution of Caenorhabditis elegans P granule condensates. Proceedings of the National Academy of Sciences of the United States of America, 118(37), e2102772118. https://doi.org/10.1073/pnas.2102772118
- Duan, X., Li, Y., & Pang, X. (2019). Protocell: A new strategy for drug delivery. Current Pharmaceutical Design, 25(29), 3099–3106.
- Eigen, M., McCaskill, J., & Schuster, P. (1988). Molecular quasi-species. The Journal of Physical Chemistry, 92(24), 6881-6891. https://doi.org/10.1021/j100335a010
- Flory, P. J. (1985). Thermodynamics of high polymer solutions. The Journal of Chemical Physics, 83(3), 1564-1570. doi: 10.1063/1.449343
- Fraccia, T. P., Iacovella, C. R., & Abbott, N. L. (2020). Liquid crystal coacervates composed of short double-stranded DNA and cationic peptides. ACS Nano, 14(11), 15071-15082. doi: 10.1021/acsnano.0c06746
- Ferris, J. P., Hill, A. R., Jr., & Liu, R. (1996). Synthesis of long prebiotic oligomers on mineral surfaces. Nature, 381(6577), 59-61. doi: 10.1038/381059a0
- Fu, K., Wu, H., & Su, Z. (2021). Self-assembling peptide-based hydrogels: Fabrication, properties, and applications. Biotechnology Advances, 49, 107752. https://doi.org/10.1016/j.biotechadv.2021.107752
- Gaspers, L. D., Bakowski, D., & Nadolski, M. J. (2017). Spatial Ca2+ Profiling: Decrypting the Universal Cytosolic Ca2+ Oscillation. Journal of Physiology, 595(10), 3053-3062. https://doi.org/10.1113/jp273212
- Gray, M. J., Wholey, W. Y., & Jakob, U. (2014). Polyphosphate is a primordial chaperone. Molecular Cell, 53(5), 689-699. doi: 10.1016/j.molcel.2014.01.025
- Gil, R. R., & Bruchez, M. P. (2015). Stability of energy metabolites - An often overlooked issue in metabolomics studies: A review. Electrophoresis, 36(17), 2156-2169. doi: 10.1002/elps.201400585
- Geiger, F. C., Kjær, J., & Andersen, K. R. (2021). Liquid-liquid phase separation underpins the formation of replication factories in rotaviruses. The EMBO Journal, 40(18), e107711. https://doi.org/10.15252/embj.2020107711
- Green, N. J., & Maréchal, A. (2021). Illuminating life's origins: UV photochemistry in abiotic synthesis of biomolecules. Journal of the American Chemical Society, 143(18), 7219-7236. doi: 10.1021/jacs.0c13200
- Hudson, J. L., Field, R. J., & Noyes, R. M. (1981). Chaos in Belousov-Zhabotinsky reaction. The Journal of Chemical Physics, 74, 6171. doi: 10.1063/1.441007
- Hyman, A. A., Weber, C. A., & Jülicher, F. (2011). Beyond stereospecificity: Liquids and mesoscale organization of cytoplasm. Developmental Cell, 21(1), 14-16. https://doi.org/10.1016/j.devcel.2011.06.013
- Hyman, A. A., Weber, C. A., & Jülicher, F. (2014). Liquid-liquid phase separation in biology. Annual Review of Cell and Developmental Biology, 30, 39-58. https://doi.org/10.1146/annurev-cellbio-100913-013325
- Ianeselli, L., Dose, B., & Maurel, M. C. (2019). Periodic melting of oligonucleotides by oscillating salt concentrations triggered by microscale water cycles inside heated rock pores. Angewandte Chemie International Edition, 58(38), 13289-13294. https://doi.org/10.1002/anie.201905005
- Kostic, D. A., Lynch, K. M., & Schubert, C. J. (2020). The second law and entropy misconceptions demystified. Entropy, 22, 648. doi: 10.3390/e22060648
- Kim, J. T., & Terman, G. (2006). Multi-scale computational model of fuel homeostasis during exercise: Effect of hormonal control. Annals of Biomedical Engineering, 35(1), 1-11. doi: 10.1007/s10439-006-9219-1
- Keil, P., Spasic, I., Utz, M., & Winterhalter, M. (2017). Proton gradients and pH oscillations emerge from heat flow at the microscale. Nature Communications, 8, 1-11. https://doi.org/10.1038/s41467-017-02065-3
- Keil, P., Spasic, I., Utz, M., & Winterhalter, M. (2016). Probing of molecular replication and accumulation in shallow heat gradients through numerical simulations. Physical Chemistry Chemical Physics, 18(30), 20153-20166. https://doi.org/10.1039/c6cp00577b
- Lukacs, G. L., Haggie, P., Seksek, O., Lechardeur, D., Freedman, N., & Verkman, A. S. (2000). Size-dependent DNA Mobility in Cytoplasm and Nucleus. Journal of Biological Chemistry, 275(3), 1625-1629. https://doi.org/10.1074/jbc.275.3.1625
- Martin, W. F., Garg, S., & Zimorski, V. (2010). Evolutionary Origins of Metabolic Compartmentalization in Eukaryotes. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1541), 847-855. https://doi.org/10.1098/rstb.2009.0252
- Mariani, A., Munn, A. S., & Swain, P. M. (2018). pH-driven RNA strand separation under prebiotically plausible conditions. ACS Biochemistry, 57(43), 1308-1315. https://doi.org/10.1021/acs.biochem.8b01080
- Martynov, V. G., & Vorob'eva, O. V. (2014). Dry polymerization of 3',5'-cyclic GMP to long strands of RNA. ChemBioChem, 15(6), 879-889. doi: 10.1002/cbic.201300773
- Mansy, S. S., Schrum, J. P., & Krishnamurthy, M. (2015). Heat flux across an open pore enables the continuous replication and selection of oligonucleotides towards increasing length. Nature Chemistry, 7(4), 315-321. doi: 10.1038/nchem.2202
- Ma, G. (2014). Microencapsulation of protein drugs for drug delivery: Strategy, preparation, and applications. Journal of Controlled Release, 193, 324–340. https://doi.org/10.1016/j.jconrel.2014.09.003
- Needleman, D., Dogic, Z., & Fraden, S. (2017). Active Matter at the Interface Between Materials Science and Cell Biology. Advanced Materials Interfaces, 4(17), 170048. https://doi.org/10.1002/admi.201700048
- Odermatt, P. D., Shrestha, R. L., & Rüdiger, M. (2021). Variations of Intracellular Density During the Cell Cycle Arise from Tip-growth Regulation in Fission Yeast. eLife, 10, e64901. https://doi.org/10.7554/eLife.6490
- Oró, J. (1960). Synthesis of adenine from ammonium cyanide. Biochemical and Biophysical Research Communications, 2(6), 407-412. doi: 10.1016/0006-291X(60)90270-4
- Oparin, A. I. (1924). The origin of life. In E. D. Fröhlich (Ed.), The origin of life on earth (pp. 71-88). New York: Macmillan.
- Patel, A. J., Varner, J. D., & Flagel, L. E. (2017). ATP as a biological hydrotrope. Science, 356(6334), 753-756. doi: 10.1126/science.aaf6846
- Perazzo, A., Preziosi, V., & Guido, S. (2015). Phase inversion emulsification: Current understanding and applications. Advances in Colloid and Interface Science, 222, 581–599. https://doi.org/10.1016/j.cis.2015.01.001
- Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines — a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261–279. https://doi.org/10.1038/nrd.2017.243
- Reinke, A., Chen, J. C., Aronova, S., Powers, T., & Cui, Y. (2006). Genomewide Oscillation of Transcription in Yeast. Trends in Biochemical Sciences, 31(4), 166-173. https://doi.org/10.1016/j.tibs.2006.01.006
- Rasmus, F. W. (2000). Darwin on variation and heredity. Journal of the History of Biology, 33(3), 425-455. doi: 10.1023/A:1004760716015
- Ritson, D. J., Sutherland, J. D., & Sutherland, J. D. (2013). Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nature Chemistry, 4(11), 895-899. doi: 10.1038/nchem.1476
- Rai, R., Alwani, S., & Badea, I. (2019). Polymeric nanoparticles in gene therapy: New avenues of design and optimization for delivery applications. Polymers, 11(4), 745. https://doi.org/10.3390/polym11040745
- Saric, A., Sitte, E., & Krause, E. (2021). Solutes as controllers of endomembrane dynamics. Nature Reviews, 22, April 2021. doi: 10.1038/s41579-020-00519-3
- Scharf, C., Virgo, N., Cleaves II, H. J., Aono, M., Aubert-Kato, N., Aydinoglu, A., … Yarus, M. (2016). Quantifying the origins of life on a planetary scale. Proceedings of the National Academy of Sciences of the United States of America, 113(29), 8127-8132. https://doi.org/10.1073/pnas.1523233113
- Schulman, R., Winfree, E., & Seeman, N. C. (2012). Robust self-replication of combinatorial information via crystal growth and scission. Proceedings of the National Academy of Sciences, 109(17), 6405-6410. doi: 10.1073/pnas.1119770109
- Seal, M., Weil-Ktorza, O., Despotović, D., Tawfik, D. S., Levy, Y., Metanis, N., Longo, L. M., & Goldfarb, D. (2022). Peptide-RNA coacervates as a cradle for the evolution of folded domains. Journal of the American Chemical Society, 144(31), 14150-14160. doi: 10.1021/jacs.2c03819
- Srimungkala, S., Noguchi, K., & Yoshida, Z. (1999). Bromination reactions important in the mechanism of the Belousov-Zhabotinsky system. The Journal of Physical Chemistry A, 103(7), 1038-1043. https://doi.org/10.1021/jp984201o
- Schuster, P. (1984). Polynucleotide evolution, hypercycles, and the origin of the genetic code. Advances in Space Research, 4(12), 143-151.
- Szathmáry, E. (2013). On the propagation of a conceptual error concerning hypercycles and cooperation. Journal of Systems Chemistry, 4(1), 1-6. https://doi.org/10.1186/1759-2208-4-1
- Tsien, R. Y., Pozzan, T., & Rink, T. J. (1982). Calcium Homeostasis in Intact Lymphocytes: Cytoplasmic Free Calcium Monitored With a New, Intracellularly Trapped Fluorescent Indicator. The Journal of Cell Biology, 94(2), 325-334.
- Uchida, S., & Kataoka, K. (2019). Design concepts of polyplex micelles for in vivo therapeutic delivery of plasmid DNA and messenger RNA. Journal of Biomedical Materials Research Part A, 107(5), 978-990. https://doi.org/10.1002/jbm.a.36614
- Wikstrom, M., Hummer, G., & Kaila, V. R. I. (2020). Thermodynamic efficiency, reversibility, and degree of coupling in energy conservation by the mitochondrial respiratory chain. Communications Biology, 3(1), 1-12. doi: 10.1038/s42003-020-01260-7
- Walter, H., Brooks III, D. E., & Fisher, E. F. (1995). Phase separation in cytoplasm due to macromolecular crowding, is the basis for microcompartmentalization. FEBS Letters, 361(2-3), 135-139. https://doi.org/10.1016/0014-5793(95)00173-3
- Wang, X., Yang, L., Chen, Z., & Wang, Z. (2019). Sol–gel immobilized biomolecules: advantages, recent developments, applications and future perspectives. Journal of Materials Chemistry B, 7(45), 7021-7031. doi: 10.1039/C9TB01772B
- Yang, L., Wang, X., & Li, Y. (2006). Crystal shape control manipulating supersaturation cooling crystalization. Crystal Growth & Design, 6(12), 2907-2915. doi: 10.1021/cg060363c
- Zhang, S., Holmes, T., Lockshin, C., & Rich, A. (2005). Supramolecular assembly of extracellular matrix glycoproteins for synthetic biomaterials. Biomaterials, 26(30), 7586-7594. DOI: 10.1016/j.biomaterials.2005.05.049.
- Zellmer, G. F., Schmidt, M. W., & Arculus, R. J. (2015). Volatiles in subduction zone magmatism. Evolution and Eruption of Arc Magmas. Geological Society of London. Special Publications, 410, 1-17. doi: 10.1144/SP410.7